Abstract
The hallmark of sickle cell disease (SCD) is polymerization of deoxygenated hemoglobin S (HbS), resulting in red blood cell (RBC) sickling, oxidative and membrane damage, hemolysis, vaso-occlusion, and end-organ damage. Exacerbating the pathogenesis of SCD, the sickle RBC (sRBC) has increased 2,3-DPG levels with decreased oxygen (O 2) affinity (increased P 50) and decreased ATP. Etavopivat, a small molecule activator of erythrocyte pyruvate kinase (PKR), increases PKR activity, resulting in decreased 2,3-DPG levels and increased ATP levels in RBCs. In a Phase 1 study in patients with SCD [NCT03815695], etavopivat significantly improved anemia and hemolysis after 2 weeks of treatment (Brown et al. Blood 2020). To evaluate the potential of etavopivat to reduce vaso-occlusive crises, exploratory studies were conducted to characterize the sRBC specific (intrinsic) and systemic effects of PKR activation.
Patients with SCD received once daily etavopivat 300 or 600 mg for 2 weeks or 400 mg for up to 12 weeks. Peripheral blood was collected prior to treatment (ie, baseline), on treatment and 7-28 days post treatment. Studies to assess the sRBC intrinsic effects of PKR activation included evaluation of RBC parameters and reticulocyte counts (ADVIA ®), membrane deformability (Lorrca ®), enzyme function studies, and membrane markers by flow cytometry. Studies to assess the systemic effects of PKR activation included markers of coagulation, inflammation, and hypoxia in the 12-week cohort only.
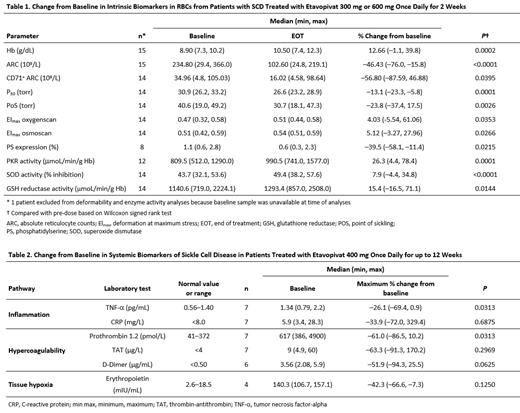
As of May 24, 2021, 15 patients who completed the 2-week dose cohorts and 7 patients treated in the 12-week dose cohort were evaluable for this analysis. The intrinsic effects of etavopivat on the sRBCs of patients receiving 2 weeks of treatment are summarized in Table 1. Etavopivat significantly increased Hb and reduced reticulocytes, including immature reticulocytes (CD71 +), suggesting that an etavopivat-mediated increase in sRBC lifespan is accompanied by a decrease in erythropoietic stress. In addition, etavopivat reduced 2,3-DPG levels thereby increasing HbS O 2 affinity (decreased P 50) resulting in a significant shift in the point of sickling (PoS) to a lower partial O 2 pressure. The deformability (EI max) of the sRBCs as measured by oxygenscan and osmoscan was significantly improved with etavopivat treatment, consistent with reduced membrane damage due to decreased HbS polymerization and improved membrane repair enabled by increased ATP production, collectively improving the health of the sRBC membrane. This improvement in membrane health was further supported by a significant reduction in the external expression of phosphatidylserine (PS) following etavopivat treatment. Finally, etavopivat significantly improved enzymatic activity not only of PKR but also superoxide dismutase and glutathione reductase, enzymes involved in reducing oxidative stress in sRBCs. This suggests that etavopivat-treated sRBCs may have an improved ability to inhibit and repair damage caused by reactive O 2 species, thereby improving overall sRBC health and function.
Initial results on the effect of etavopivat on systemic biomarkers that are commonly elevated in SCD are shown in Table 2. In patients receiving etavopivat 400 mg once daily for up to 12 weeks, tumor necrosis factor-a and prothrombin 1.2, as systemic markers of inflammation and hypercoagulability, respectively, showed a significant decrease compared with baseline. Furthermore, a trend towards reduced erythropoietin levels suggests that etavopivat treatment may reduce tissue hypoxia.
Patients with SCD treated with etavopivat for at least 2 weeks demonstrated a significant increase in RBC membrane deformability and improved antioxidant capacity that resulted in increased sRBC survival and decreased anemia. The reduced reticulocyte count and lowered PS surface membrane expression suggest that etavopivat-treated sRBC may have reduced adhesive properties and may thus be less likely to promote vaso-occlusion. Initial studies evaluating the downstream effects of up to 12 weeks of etavopivat treatment once daily provided evidence for a reduction in markers of inflammation and hypercoagulability, with improved O 2 delivery capacity. These initial results suggest that the multimodal effects of decreased 2,3-DPG and increased ATP by PKR activation with etavopivat may have an impact on both the anemia and vaso-occlusive events that characterize SCD.
Kalfa: Agios Pharmaceuticals, Inc.: Other: Steering Committee, Research Funding; FORMA Therapeutics, Inc: Research Funding. Telen: GlycoMimetics, Inc.: Consultancy; Novartis, Inc.: Other: Data Safety Monitoring Board; Forma Therapeutics, Inc.: Consultancy, Research Funding; CSL Behring, Inc.: Research Funding; Doris Duke Charitable Foundation: Research Funding; National Institutes of Health: Research Funding. Saraf: Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Research Funding. Brown: Novo Nordisk: Consultancy; Imara: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Global Blood Therapeutics: Consultancy, Research Funding; Pfizer: Research Funding; Forma Therapeutics: Research Funding. Larkin: Forma Therapeutics, Inc.: Research Funding. Ribadeneira: Forma Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Schroeder: Forma Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Wu: Forma Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Kelly: Forma Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Kuypers: Forma Therapeutics, Inc.: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal